Together we can prevent vision loss
Odylia is developing a gene therapy to treat vision loss caused by RPGRIP1 mutations, for which there is currently no treatment. This gene therapy uses the novel Anc80 vector technology developed by Odylia co-founder, Luk Vandenberghe, and builds upon proof-of-concept data generated at Massachusetts Eye and Ear in the labs of Eric Pierce and Luk Vandenberghe. Odylia is currently conducting late-stage preclinical experiments and is preparing for IND submission for use of the gene therapy in clinical trials. To advance the program towards IND, grant funding from the Foundation Fighting Blindness supports manufacturing and toxicology testing. Odylia is actively looking for partners to bring this program to clinical trials in partnership with Odylia. The program has Orphan Drug and Rare Pediatric Disease Designations.
Odylia is exploring hybrid models of funding which include donor support as well as private/commercial investment. We are committed to moving this gene therapy forward to ultimately prevent vision loss and are actively seeking partners and donors at this time. For more information on how you can support Odylia follow the link. If your company may be interested in becoming a please send an email to hgreene@odylia.org
LCA6 Disease Overview
Leber congenital amaurosis (LCA) is a type of inherited eye disorder leading to vision loss at birth or in early childhood.
- Accounts for 20% of blindness in school-age children
- Vision-loss onset often seen at birth
- Symptoms can include: profound vision loss, involuntary eye movements (nystagmus), crossed eyes (strabismus), and sensitivity to light (photophobia)
At least 27 different genes are associated with LCA. Mutations in both copies of the RPGRIP1 gene cause a spectrum of vision-related deficits most commonly diagnosed as LCA type 6 (LCA6). LCA6 patients are often completely blind from early childhood and have a rapid decline in function of the photoreceptor cells of the retina. The RPGRIP1 protein is needed for the normal function of the photoreceptors (rods and cones) of the retina, and loss of this protein is what causes the vision loss in patients. Vision loss caused by RPGRIP1 mutations is particularly devastating because of the rapid onset and progression.
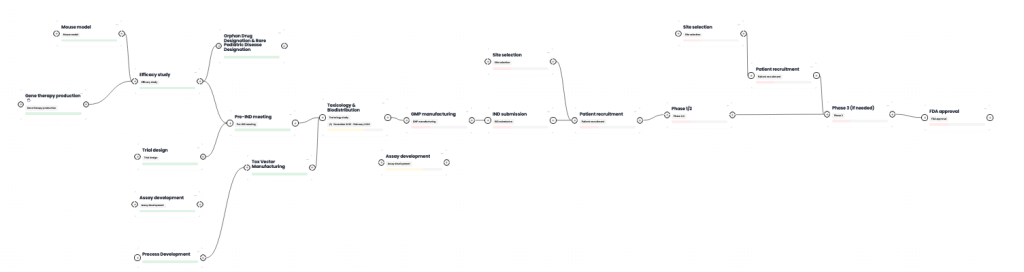
Program Resources at a Glance
NEW! Now you can track OT-004 progress on the Comend Platform. Click the image below to learn more.
Community Q&A and Program Updates
RPGRIP1 Gene Therapy Program- Community Q&A (December 2025)
RPGRIP1 Gene Therapy Program- Community Q&A (March 2025)
RPGRIP1 Gene Therapy Program- Community Q&A (June 2024)
RPGRIP1 Gene Therapy Program- Community Q&A (November 2023)
RPGRIP1 Gene Therapy Program- Community Q&A (May 2023)
RPGRIP1 Gene Therapy Program- Update (February 2022)
Clinical Advisory Group
Odylia has assembled a group of clinical thought leaders in the field of subretinally administered gene therapies.

Robert K. Koenekoop MD, MSc, PhD, FRCS(C), FARVO
Professor of Paediatric Surgery, Human Genetics and Adult Ophthalmology at McGill University
Director of the Laboratory for Retinal Genetics and Therapeutics
Chief Paediatric Ophthalmology
Montreal Children’s Hospital

Jiong Yan, MD
Associate Professor of Ophthalmology
Director, Vitreo-Retina Surgery Fellowship
Emory University School of Medicine

Tiansen Li, PhD
Senior Investigator, Retinal Cell Biology and Degeneration Section
National Eye Institute

Eric A. Pierce, MD, PhD
Director, Inherited Retinal Disorders Service, Massachusetts Eye and Ear
William F. Chatlos Professor of Ophthalmology, Harvard Medical School
Massachusetts Eye and Ear

Thaddeus (Ted) Dryja, MD
Professor of Ophthalmology, Harvard Medical School
Physician and Surgeon, Massachusetts Eye and Ear Infirmary
Massachusetts Eye and Ear Infirmary
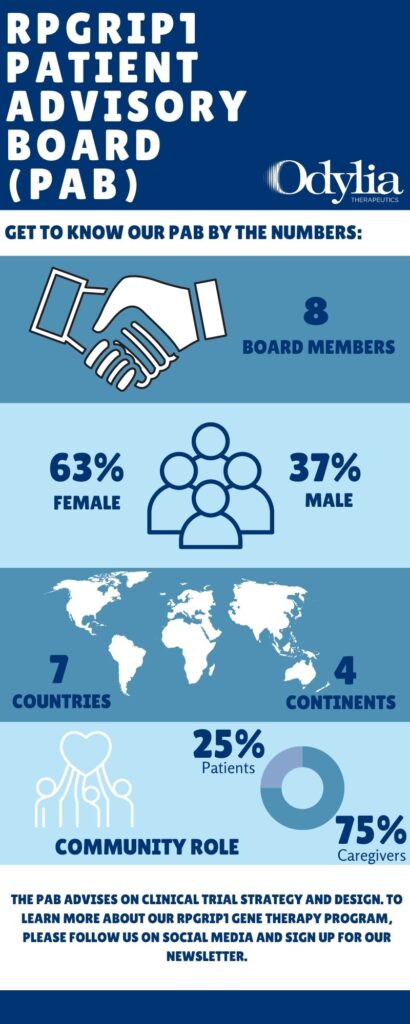
Patient Advisory Board
Odylia Therapeutics has assembled a Patient Advisory Board (PAB), with 8 members representing 7 countries on 4 continents, to assist in the development of a clinical plan for our RPGRIP1 gene therapy. The PAB provides critical insight into the patient and caregiver experience and Odylia will utilize these insights as we develop a strategy for a future clinical trial. To learn more and stay informed, please sign up for our newsletter.