Odylia Gene Therapy Pipeline
Odylia is continuously evaluating new opportunities to add to our rare disease pipeline. If you have a therapeutic already in development that you believe would be a great match for Odylia, please reach out to us. We can evaluate the potential for integrating your therapeutic or gene target into the Odylia pipeline.
Below is more information about our active pipeline programs, RPGRIP1, USH1C, and NPHP1 (details coming soon).
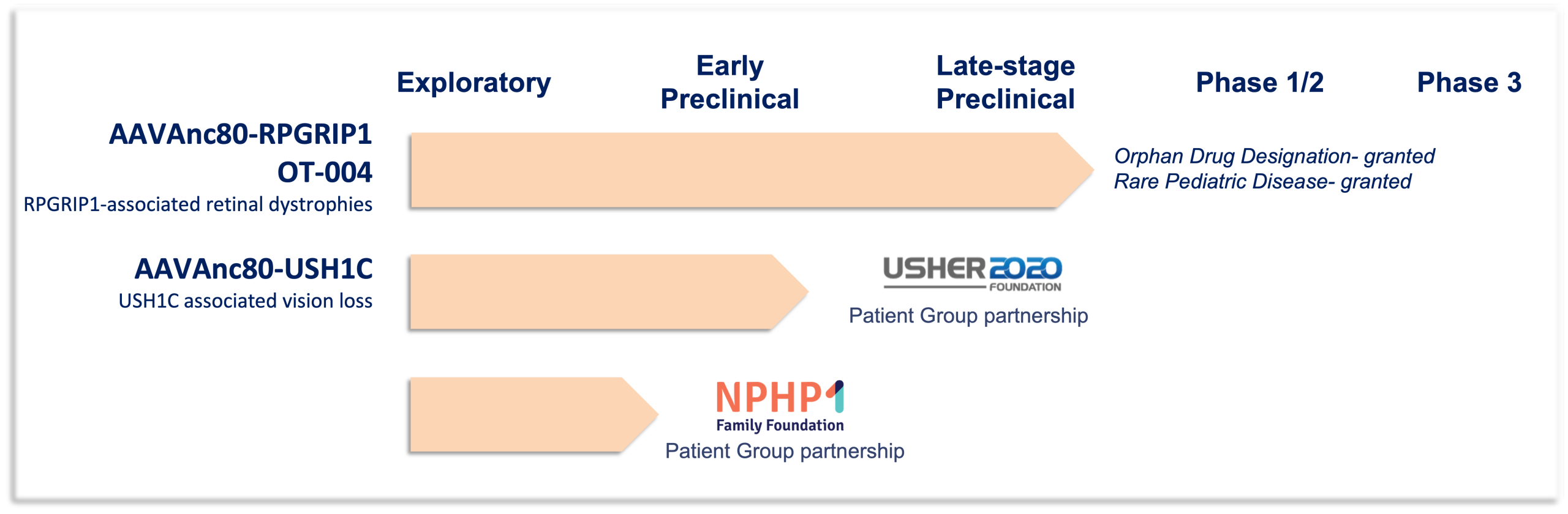
RPGRIP1 -associated retinal dystrophies (including LCA6, CORD13, juvenile Retinitis Pigmentosa)
Unpartnered, funding support from Foundation Fighting Blindness and donations
Leber congenital amaurosis (LCA) is one of the most severe retinal diseases that leads to vision loss, accounting for 20% of blindness in school-age children. LCA symptoms can develop as early as birth beginning with profound vision loss and involuntary eye movements, also known as nystagmus. Mutations in both copies of the RPGRIP1 gene cause a spectrum of vision-related deficits most commonly diagnosed as LCA type 6 (LCA6). Vision loss caused by RPGRIP1 mutations is particularly devastating because of the rapid onset and loss of vision. LCA6 patients are often completely blind from early childhood. There are currently no treatments for LCA6. In 2019 PTC Therapeutics licensed from Odylia an investigational gene therapy treatment for RPGRIP1 patients. This gene therapy uses the novel Anc80 vector technology developed by Odylia co-founder, Luk Vandenberghe, and builds upon proof-of-concept data generated at Massachusetts Eye and Ear in the labs of Eric Pierce and Luk Vandenberghe.
Treatment of vision loss, Usher Syndrome Type 1 caused by USH1C mutations
Partners: Usher 2020 Foundation, FAUN Foundation
Usher syndrome accounts for 50% of all inherited deaf-blindness disorders. It is characterized by profound deafness, progressive vision loss, and vestibular dysfunction with varying ages of initial symptom onset and severity. Usher Syndrome Type 1 is the most severe form of Usher Syndrome. Type 1 patients are born profoundly deaf, often have delayed motor skills, and have early-onset vision loss caused by retinitis pigmentosa. Inheritance of mutations in both copies of the USH1C gene cause Usher Syndrome Type 1. Oftentimes vision loss associated with USH1C mutations begins around 10 years of age with initial onset of night-blindness and a loss of side (peripheral) vision due to the dysfunction and eventual death of the light sensing cells of the retina, the photoreceptors. Vision loss progresses to tunnel vision, or loss of all but the most central portion of the visual field. There are currently no treatments for the vision loss in Usher syndrome caused by USH1C mutations. Odylia has partnered with two patient advocacy groups, the Usher2020 Foundation and the FAUN Foundation, to develop a novel USH1C gene therapy. We are collaborating with scientific experts around the world, including Germany, the Cech Republic, and the United States, to streamline development of this gene therapy.
NPHP1 Gene Therapy
Treatment of vision loss caused by NPHP1 mutations
Partners: NPHP1 Family Foundation
Mutations or deletions of the NPHP1 gene lead to a kidney condition called nephronophthisis. Nephronophthisis, a ciliopathy, is the leading cause of end-stage renal disease (ESRD) in children. While nephronophthisis is primarily a disease of the kidney, and thus, much research and attention is focused on the renal phenotype,10- 15% of patients also present with extrarenal manifestations, the most common of which is retinal dystrophy. Vision loss often progresses slowly, but both the severity and rate of progression vary from person to person.
There are currently no approved therapies to correct or prevent vision loss caused by NPHP1 mutations. A treatment would transform the quality of life and future of patients suffering with vision loss caused by these mutations, as well as for those patients that have not yet begun to lose vision. Collaborating with a leading expert on NPHP1 cell biology, Dr. Friedhelm Hildebrant and his lab at Boston Children’s Hospital, Odylia and the NPHP1 Family Foundation have defined a path forward to quickly reach pre-IND submission in 2027.




